Shenzhen Alu Rapid Prototype Precision Co., Ltd.
Industry News
- Home
- News
- How to medical device prototyping?
Here’s a practical, step-by-step guide to medical device prototyping, based on real-world practices used by startups, established medtech companies, and regulatory consultants. This process balances speed, cost, safety, and eventual regulatory compliance (ISO 13485, FDA 21 CFR 820, EU MDR).
Phase 1: Define Requirements & Risk Early (Most Important, Often Skipped)
A.Write a short Product Requirement Document (PRD)
Intended use, user needs, target patient population
Clinical claims (diagnostic, therapeutic, monitoring, etc.)
Risk classification (FDA Class I, IIa, IIb, III or EU MDR Class I, IIa, IIb, III)
B.Perform preliminary risk management (ISO 14971)
Identify hazards (electrical, biological, usability, etc.)
This drives almost every material and design decision later.
Phase 2: Concept Prototyping (Proof-of-Concept, “Looks-Like” + “Works-Like”)
Goal: Validate the core technical principle cheaply and fast.
Phase 3: Alpha Prototypes (Integrated, “Works-Like + Looks-Like”)
Common methods:
A.High-end 3D printing
B.CNC machining (aluminum, PEEK, titanium) for metal parts
C.Custom PCBs (KiCad → JLCPCB or domestic fab for speed)
D.Silicone overmolding (Smooth-On Dragon Skin or biocompatible equivalents) for soft parts
E.Rapid injection molding (Protolabs, Xometry “on-demand molding”) – 5–25 parts in real material
Phase 4: Beta / “Design Freeze” Prototypes
Looks and functions essentially like production.
A.Use production-intent processes whenever possible
B.Build 20–100 units (depends on class and risk)
C.Perform formal verification & validation (V&V)
Phase 5: Pilot Production & Design Transfer
A.Final design locked → Design History File (DHF) complete
B.Process validation (IQ/OQ/PQ) for critical processes
C.First clinical investigation or 510(k)/De Novo/PMA submission package
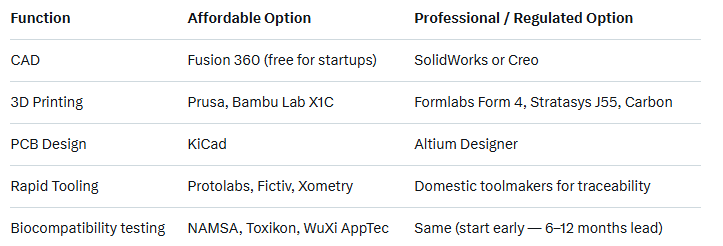
Recommended Toolchain (2025)
Critical Regulatory Tips for Prototyping
A.Document everything in your Design Controls (even for prototypes) — FDA will ask for it.
B.If you ever touch a human or animal, you need IRB/IEC approval and documented biocompatibility (ISO 10993).
C.Keep a “prototype log” — what changed and why — this becomes your design history.
D.Avoid “clinical use” of non-sterile, non-biocompatible prototypes — huge regulatory red flag.
