Shenzhen Alu Rapid Prototype Precision Co., Ltd.
Industry News
- Home
- News
- How to medical device prototyping?
Medical device prototyping is a highly specialized, iterative process that differs significantly from regular consumer product prototyping due to strict safety requirements, biocompatibility concerns, and heavy regulatory oversight (FDA in US, EU MDR/CE marking in Europe, etc.).
Typical Stages of Medical Device Prototyping
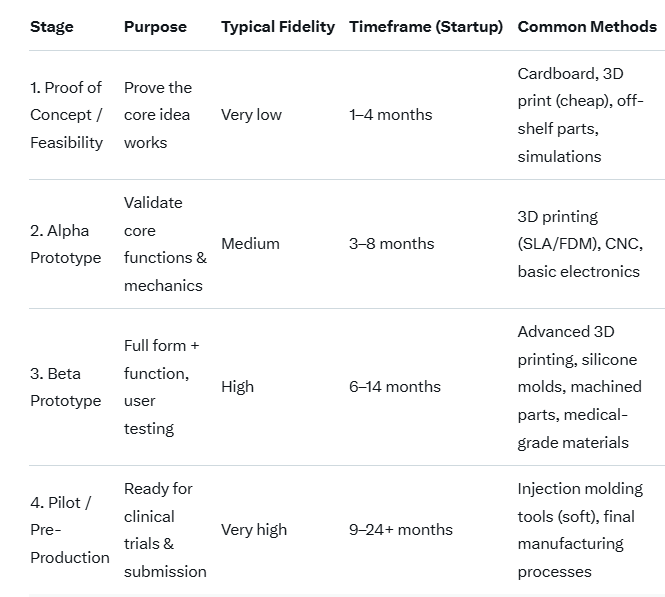
Most Popular Prototyping Methods in 2025–2026 (Medical Field)
1.3D Printing (still #1 for speed)
SLA (stereolithography) → best surface finish & accuracy
MJF / SLS → good for functional nylon parts
Medical-grade resins (biocompatible, sterilizable) now widely available
Very fast iteration (days instead of weeks)
2.CNC Machining
Best for: metal parts, tight tolerances (±0.01 mm), real engineering plastics (PEEK, Ultem, Radel)
Medical-grade metals: 316L stainless, titanium, CoCr
3.Silicone/Urethane Casting (soft tooling)
Bridge between 3D printing and injection molding
20–150 pieces with production-like appearance & feel
4.Injection Molding (rapid/aluminum tools)
Used earlier than most people think (already at beta stage for serious projects)
Critical for final material validation & regulatory submission
5.Hybrid approaches (most common reality)
3D printed housing + CNC machined critical components + commercial off-the-shelf electronics
Critical Regulatory & Material Considerations (Don't Skip These!)
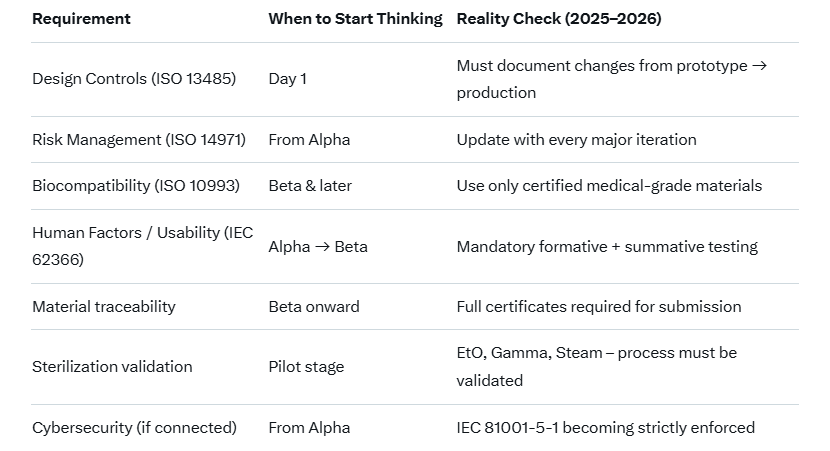
Realistic Advice for Startups (2025–2026)
1.Budget reality
Good functional medical prototype journey → usually $80k–$400k+ (depending on complexity)
2.Fastest realistic timeline (very disciplined team)
Concept → working Beta prototype ready for early clinical feedback → 12–20 months
3.Most common fatal mistakes (avoid them!)
Using non-medical-grade materials too long → big re-design later
Ignoring usability/human factors until too late
Switching manufacturing method at pilot stage without bridging data
No design history file / poor documentation
Trying to do everything in-house without medical-grade manufacturing experience
4.Smart startup strategy in 2025
Phase 1: Cheap & fast (3D print + off-shelf) → prove concept & get funding
Phase 2: Partner with experienced medical prototyping company (they know ISO 13485 workflow)
Phase 3: Early regulatory consultant (huge time & money saver)
